•
Write the author
With the enactment of Patents (Amendment) Act 2005 the amending process of Indian Patents Act 1970 to bring it in line with the TRIPS
Agreement has been completed by the government. The earlier two amendments were enacted by Parliament during 1999 and 2002. In the
amending process some safeguard provisions have been incorporated. However, still some more possibilities in this direction within the
framework of the TRIPS Agreement have been ignored. In addition there are a few others stipulations which need to be rectified to avoid
legal disputes. The original Patents Act 1970 was a balanced Act which helped the growth of industry and also adequately covered the
public interest angle. The pharmaceutical industry produces high quality products of almost all therapeutic groups and exports the
generic produce to the developing and developed countries at most competitive prices. The developing countries are now apprehending
difficulties in importing pharmaceuticals from India because of the tight provisions in regard to the compulsory licences for effective
role of the domestic enterprises in the patented products.
Public Response
The National Working Group on Patent Laws has been deliberating on various WTO Agreements including the TRIPS Agreement ever since 1988
when the Uruguay Round of GATT Negotiations were in full swing. Its main objective has been to provide studies for informed debate in
Parliament and in public on the Uruguay Round Agenda / Final Act of WTO on availability and affordability of medicines. The group has
also been organizing national and international seminars/conferences. The National Working Group on Patent Laws also established four
Peoples' Commissions on TRIPS issues. The first Commission was established to deal with the constitutional issues of the Final Act of WTO
including TRIPS Agreement in November 1993 with the Chairman and the members being eminent former Judges of Supreme Court of India. Again
in 1999 another Commission was established to deal with the transitional period obligations in the TRIPS Agreement. The Chairman of this
Commission was also former Judge of Supreme Court and members were senior scientists and economists.
The Third Peoples' Commission was established during 2002 on appropriate patent law for India. The Chairman of this Commission was the
former Prime Minister of India and the members were eminent and senior experts. The Fourth Peoples' Commission was established on review
of patent legislations in February 2004, again with the former Prime Minister of India as the Chairman and a number of senior and eminent
experts as members. Reports of all these Commissions were submitted to government and also made available to all political parties and
Members of Parliament. No opportunity was provided to these Commissions for discussion with the government. In addition to these reports
there were, as stated earlier, a number of other publications which were made available to the concerned Ministries of the government and
to the Members of Parliament. All these efforts are being pointed out to bring home that the process of amending the patent law to
fulfill obligations to bring the patent law in line with the TRIPS Agreement has been completed without adequate deliberations between
the government and the public.
Consideration by Parliament
The original Patents Act 1970 was a balanced Act which helped the growth of industry and also adequately covered the public interest
angle. The pharmaceutical industry produces high quality products of almost all therapeutic groups and exports the generic produce to the
developing and developed countries at most competitive prices The developing countries are now apprehending difficulties in importing
pharmaceuticals from India because of the tight provisions with regard to the compulsory licences for effective role of the domestic
enterprises in the patented products.
The NDA government introduced the final amending Bill in the Lok Sabha (Lower House) in December 2003 and the Bill was referred to the
Parliamentary Standing Committee on Commerce. This Bill however, lapsed due to general elections to the Lok Sabha. The new UPA government
promulgated an Ordinance in December 2004. The Ordinance was almost an exact copy of the December 2003 Bill. The Ordinance was to be
replaced by a formal enactment of the Bill and the same was introduced in the Lok Sabha in March 2005. Again the Bill was the copy of the
Ordinance. UPA government and the Left parties negotiated certain important amendments and the same were brought forward as government
amendments. There was then no difficulty in passing the Bill during the debate in both the houses. The opposition members stage walkouts
in both the Houses. The Bill could have been referred to the Standing Committee of Parliament as its provisions were to be effective from
1.1.2005 as stipulated in Article 1 of the Bill. The committee could have provided opportunity to outside experts to project some other
important amendments left out. However, the government managed to avoid referring of the Bill to the Standing Committee of Parliament.
The above would show that the process of amendment of the Patents Act 1970 was more guided by the political compulsions. Thus there was
no national consensus in amending the Patents Act 1970. As pointed out earlier there are still a few important provisions which have been
ignored in the amending process. The government has now set up a technical expert group to look into two specific amendments relating to
the definition of 'pharmaceutical entity' and exclusion from 'patentability of micro-organisms'. The flexibilities and other aspects
which require consideration are dealt with in Part III of this paper. It is also hoped that Technical Expert Group would invite
stakeholders for evidence.
Patent system for India
Patent system is not new to India. The first patent law was enacted in 1858. A comprehensive law was, however enacted by the British
rulers in 1911. This act was designed to serve the foreign interests and for control over markets for finished goods by multi-national
corporations. In so far as pharmaceutical products were concerned over a period almost 85 per cent of medicines were supplied by the
multi-national corporations. Kefauver Committee of USA which deliberated extensively on the availability of medicines worldwide and the
role of the multi-nationals pointed out in their report that the prices of antibiotics and other medicines in India were the highest in
the world. The Indian people were virtually fleeced on the availability and affordability of medicines.
National Patents Act 1970
Immediately after Independence in 1947 our leaders were seriously concerned about the enactment of a national patents system relevant to
the stage of our development. The objectives were that there should be faster industrialization of the country and law should be designed
to serve the public interest in a balanced manner. Two important committees headed by Justice Bakshi Tek Chand and Justice Rajagopal
Iyenger dealt with the patent law issues relevant for our country in their reports. Based upon the recommendations in these reports a
comprehensive Patents Bill was framed and debated extensively in parliamentary committees and both Houses of Parliament. Finally the
National Patents Act was enacted in 1970. This law served the objectives which our leaders had in view.
With the passage of time the Indian companies in pharmaceutical field grew at a fast pace and their share of market in the availability
of medicines went up to about 85 per cent. As regards the prices of medicines due to competitive environment and because of the new
patent system the prices of medicines became the lowest in the world. The industry has also developed enough surplus capacity to meet
export demands from the developed and developing countries.
The main features of the Patents Act 1970 were as follows :
There was no product patent for pharmaceuticals, food and chemical based products. These industrial sectors were covered by process
patent only.
The term of the patent was 7 years from the date of application or 5 years from the date of sealing of patent which ever period was
lower.
In order to ensure pronounced role of the domestic enterprises in the patented product a system of 'licensing of right' was also provided
for the sectors covered by the process patent.
There was no constraints on exports.
The patent holder was under obligation to use the patent. There was also provision for revocation of patent for non-use.
For licences of right the royalty ceiling was stipulated at 4 per cent.
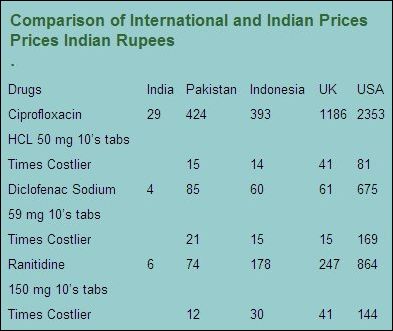
The following chart indicates how the prices of medicines in India are the lowest as compared to those in other
countries.
TRIPS patents system
The TRIPS Patents System is based upon a joint statement (paper) presented by the multinational associations of USA, Europe and Japan to
the GATT Secretariat in June 1988 during the Uruguay Round Negotiations. The main features of the TRIPS patent system are as follows :
TRIPS provides for patent protection for any inventions whether products or processes in all fields of technology provided that they are
new, involve an inventive step and are capable of industrial application.
The foreign patent holders have been absolved from working of their patents and imports by them are to enjoy the same patent rights
without discrimination as to the place of invention, field of technology and whether the products are imported or locally produced.
The term of all patents shall not end before the expiration of 20 years from the date of application.
There is no 'licensing of right' provision. The compulsory licence provisions are having tight conditionalities with constraints for
exports.
There is no royalty ceiling for compulsory licences. The royalty payment is based on the economic value of the licence.
The above features of the TRIPS Agreement have been implemented in the amending process of our Patents Act 1970
Doha Declaration Ignored
The approach of amending the Patents Act 1970 to bring it in line with the provisions of the TRIPS Agreement should have been carefully
worked out so that all flexibilities available in the TRIPS Agreement which were also clarified in the Doha Declaration on TRIPS
agreement and public health had been implemented with utmost precision. Certain important flexibilities of TRIPS ignored relate to those
provisions which are stipulated in the following Articles :
Article 7 - Transfer of Technology & Balancing of Rights and Obligations
Article 8 - Promotion of public interest in sectors of vital importance.
Article 27.3 (b) - Patenting of micro-organisms and non-biological and micro-biological processes.
Article 31(b) - Compulsory licence for commercial activity on reasonable terms & conditions.
Article 70.3 - Mail Box products in public domain as on 1.1.2005.
The WTO agreements have drawn widespread protests not only from the people living in the developing world but also from many an activist
groups in the affluent west
Recent international studies ignored
There have been several important studies in recent past on implications of the TRIPS Agreement. These studies should have served as a
guide to frame amendments to Indian patent law. These studies are :
The gist of these studies relate to wide range of questionable inventions patented in USA, having only incremental modifications which
are discouraging generic companies and blocking of competitive products into USA. Another situation which is emerging in USA is about the
flood of patent applications rising to over 300 thousands annually resulting into grant of poor quality or questionable patents. UK
Commission on IPR made specific recommendations to contain patentable subject matter by defining patentable inventions and other
terminologies appropriately.
These studies thus are extremely useful pointers to be careful in dealing particularly about the need to determine the scope of
patentability and freedom to determine proper definitions of 'invention' and other terminologies which are extremely important for
amendments to Indian Patents Act to bring the same in line with the TRIPS Agreement.
It is pertinent to note that economists of repute who otherwise are fully supportive of the free trade theory and the WTO ( Jagdish
Bhagwati, Dani Rodrik, Michael Finger) have, of late, recognized the inequity of the TRIPS agreement from the point of view of developing
countries and some have even questioned the logic of incorporating TRIPS into the WTO system in the first place.
Even a large number of mass organizations and international organisations have pointed out to the government authorities in India to use
flexibilities in their laws so that the countries dependent upon supplies of pharmaceuticals are not deprived of imports from India.
Dr. Yusuf K Hamied, Chairman and Managing Director of Cipla Limited and a leading scientist in his recent Paper 'Trading in Death' has
made strong observations on the new Indian Patent Law keeping the critical health scenario in India in view :
"The truth is that health in India is in a permanent and perpetual crisis. The disease profile is as follows : 80 million cardiac
patients, 80 million afflicted with mental illness, 60 million diabetics, 50 million asthmatics, 50 million hepatitis B cases, and one in
three Indians is a latent carrier of TB. The World Bank has said that India will have 35 million HIV cases by 2015, or approximately half
of all the AIDS cases in the world. Given these facts, the patent regime in this country should be devised so that the utmost priority is
granted to securing the people's rights of access to affordable and quality healthcare, without monopoly".
Keeping the views expressed the important provisions of the Amended Patents Act 1970 which need to be reviewed and amended are dealt with
as follows:
Scope of Patentability
Section 2 : Definitions and Interpretations
1. Clause (j) defines invention as follows :
- 'invention' means a new product or process involving an inventive step and capable of industrial application;
In order to limit patentable subject matter it is suggested that the definition of invention could be changed as follows :
'invention' means a basic new product or process involving inventive step and capable of industrial application.
The U.K. Commission on I.P.R. has also recommended that developing countries should aim at limiting the subject matter of patents. Even
increasing volume of patent claims going up over 300 thousand in USA and China - claiming patents even for minor incremental changes etc.
The scope of patentable inventions can be contained only through the definition of invention.
2. Clause (ta) defines pharmaceutical substance as follows :
- 'pharmaceutical substance' means any new entity involving one or more inventive steps.
The above definition is quite broad and not specific. Even Mashelkar Committee on R&D for pharmaceuticals had made re-commendation in
this respect. The definition aught to be as follows :
(ta) 'pharmaceutical substance' includes new drug molecule involving one or more inventive steps.
What is not patentable
3. Clause (d) reads as follows :
- the mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that
substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or
apparatus unless such known process results in a new product or employs at least one new reactant.
The explanation under clause (d) reads as follows :
Explanation :- For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers,
mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance,
unless they differ significantly in properties with regard to efficacy.
The above explanation can give different interpretations for different purposes. Moreover when new molecules only are
to be patentable salts, esters, etc. and other derivatives of the known substance should not be treated as patentable inventions. The
purpose of clause (d) and explanation should be to contain the patentable subject matter. The explanation should be either deleted or
modified as follows: Explanation :- For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle
size, isomers, complexes, combinations and other derivatives of known substance shall not be patentable.
4. New clause (ja) may be incorporated as follows :
- inventions which do not strictly meet the criteria of industrial application e.g. onco mouse, stem cell, partial gene fragments,
research tools, PCR technique, machine based embedded bio-informatics software, genomic information and data base.
5. Clause (j) in the Patents Act reads as follows:
- plants and animals in whole or any part thereof other than micro-organisms but including seeds, varieties and species and essentially
biological processes for production or propagation of plants and animals.
Micro-organisms and non-biological and micro-biological processes are under mandated review in the WTO. The review was initiated in 1999
and till now final decision has not yet been taken. In addition to this no definition of micro-organism has been provided in the amended
Act or even in the TRIPS Agreement. Micro-organisms occur in nature, there are genetically modified micro-organism and then they perform
certain activities. Since micro-organism occurs in nature they are discoveries and not inventions and as such they should not be
patentable. Genetically modified micro-organisms perform certain activities and as such only specific activity can be patented only as
process patents. Because of these reasons it is desirable that micro-organisms are excluded from patentability. As an alternative since
provision has been made in the amended Patents Act the implementation of patentability of micro-organism may be postponed till the
decision has been taken in the WTO on mandated review. The date of its implementation may be notified at the appropriate time.
6. Section 5: The amended Act provides that Section 5 shall be omitted. However, it is important to be more specific about the scope of
patentability. Because of the new scope of patentability Section 5 should read as follows :
Section 5 - Patents shall be available for basic new inventions including pharmaceutical substances as defined in Section 2 clause (ta)
whether products or processes in all field of technologies provided that they are new, involve an inventive step and are capable of
industrial application excluding inventions not patentable as stipulated under Section 3.
7. Section 11 A Sub-section (7)
The third proviso of this sub-section reads as follows :
- provided also that after the patent is granted in respect of applications made under sub-section (2) of section 5, (Mail Box
applications filed during 1995-2004) the patent holder shall only be entitled to receive reasonable royalty from such enterprises which
have made significant investment and were producing and marketing the concerned product prior to the 1st day of January 2005 and which
continue to manufacture the products covered by the patent on the date of grant of the patent and no infringement proceedings shall be
instituted against such enterprises.
Article 70 para 3 of TRIPS stipulates as follows :
- There shall be no obligation to restore protection to subject matter (i.e. mail box applications) which on the date of application of
this Agreement for the Member in question (i.e. 1.1.2005) has fallen into the public domain.
The above stipulation in the TRIPS Agreement clearly provides that any mail box product which has fallen in the public domain as on 1st
day of January 2005 should not be patentable.
The stipulation in the provison stated above could mean that we are making more stringent provision than the TRIPS stipulation. This
matter needs to be seriously considered and no patent protection provided for such products. Thus the existing manufacturers can continue
production.
Even the provisions in sub-clause (7) about 'reasonable royalty' and 'significant investment' can create unnecessary claims and
objections to meet the objectives of allowing enterprises to continue manufacturing of the product if the patents are granted. In
addition the applications should be dealt with only on the basis of proposed new definitions of invention and pharmaceutical substances.
8. Section 25
Section 117 A in sub-section 2 provides for sections for which appeals shall lie to the appellate board. The amended version of this
section provides that only sub-section (4) of Section 25 relating to post-grant opposition would be appealable. No appeal possibility is
thus available in regard to pre-grant opposition. It is important that even any decision taken relating to pre-grant opposition should
also be appealable both by the patent applicant and the opponent. In view of this, it would be appropriate if decisions taken under the
entire Section 25 become appealable.
9. New Section 84 A
Article 31(b) of the TRIPS Agreement clearly stipulates that the member can allow the use of the subject matter of a
patent provided that: (b) such use, may only be permitted if, prior to such use, the proposed user has made efforts to obtain
authorization from the right holder on reasonable commercial terms and conditions and that such efforts have not been successful within a
reasonable period of time. Based on the above stipulation in the TRIPS Agreement a large number of countries (developed and developing)
have made specific compulsory licence provisions in their patent laws. As examples, the provisions in the patent laws of Brazil and China
are reproduced as follows :
BRAZIL
The Patents Act of Brazil provides for compulsory licence for exploitation of the patent as follows :
Article 73. An application for a compulsory licence shall be drawn up by setting out the conditions offered to the patent owner.
(1) On filing of the licence application, the patent owner shall be invited to submit his comments within a period of 60 days, on expiry
of which, in the absence of a reply from the patent owner, the proposal shall be deemed accepted under the conditions offered.
CHINA
The Patents Act 1992 of China provides for Compulsory Licence for exploitation of the patent as follows :
Article 51. Where any entity which is qualified to exploit the invention or utility model has made requests for authorization from the
patentee of an invention or utility model to exploit its or his patent on reasonable terms and such efforts have not been successful
within a reasonable period of time, the patent office may, upon the application of that entity, grant a compulsory licence to exploit the
patent for invention or utility model.
It is important to point out that world over the important International Organisations, Mass Organisation in South East Asia and American
and European Continents are perturbed over the amendments made to the Patents Act 1970 without the provision in Section 84A. Even
important News Papers in their editorials and articles by world known economists have commented upon the seriousness of the situation
which might emerge from the amended Indian Patents Act.
Section 84 A
1. When the proposed user has made efforts to obtain authorization from the patentee to use the patent on reasonable commercial terms and
conditions and that such efforts have not been successful within a period not exceeding 100 days, the controller shall at any time after
the date of grant of patent grant compulsory licence to the applicant on such terms and conditions as he may deem fit.
2. The commercial terms and conditions offered by the applicant shall be considered reasonable by the controller if royalty and other
remuneration offered by him are within five per cent of the annual sale turnover at net ex-factory sale price (exclusive of excise duty
and sales tax).
It is important to point out that world over the important international organisations, mass organisation in South East Asia and American
and European continents are perturbed over the amendments made to the Patents Act 1970 without the above provision in Section 84A. Even
important newspapers in their editorials and articles by world known economists have commented upon the seriousness of the situation
which might emerge from the amended Indian Patents Act affecting the availability and affordability of medicines in the poor country who
are dependent upon exports from India. The concerns have been expressed because certain TRIPS flexibilities concerning public interest
and particularly the role of the domestic enterprises have been ignored. The concerns have been raised by the World Health Organisation,
Geneva, UNAIDS, Geneva, Special Envoys of the UN Secretary General for HIV/ AIDS in Asia and the Pacific and in Africa and International
Council of Medecins Sans Frontiers, Geneva. Their concern can be satisfied only when the suggested provision in Section 84A is
implemented. It seems the government has missed this provision under pressure and provided the same as a condition under Section 84
dealing with compulsory licences due to abuse of patent rights by the patentee which is clearly a misplaced provision.
10. Section 90, Sub-section 1 clause (vi)
provides for a shorter term for the compulsory licence. No one would be interested to take compulsory licence for a shorter period and
hence shorter term may be deleted. Moreover no basis can be provided or determined to provide for shorter term.
11. Section 92
Circumstances of extreme urgency may be defined as notified 'health emergency' in the country as whole or in a region and environmental
emergency relating to soil, water or air pollution limited to the region or the country as a whole.
12.Provision about 'right of priority' should be expanded to provide that in regard to pharmaceutical and agro-chemical right of priority
will be only upto 1.1.1995 and not for an earlier date. As regards the other products where product patent has been provided as from
1.1.2005 right of priority should not be applicable prior to 1.1.2005. Non-provisioning of these aspects will bestow unnecessary
advantage to the patent applicants.
Price Control
TRIPS Agreement is silent about the price control of patented products. The products protected under patents would enjoy monopoly in the
market place and would certainly command high prices. Appropriate law should be strengthened to deal with the prices of the patented
products at least for the initial period of 5 years. The importance of this aspect can be understood on the basis of example of prices of
similar product in India, Pakistan and Indonesia. A pack of ten 500 mg tablets of Ciprofloxacin cost Rs. 29 in India whereas the price in
Pakistan is Rs. 424 and in Indonesia it is Rs. 393 (converted to Indian Rupees). The prices of other pharmaceutical products are also
almost in the similar proportion.
Conclusions
The originators of inventions should get their just reward by way of suitable royalties and there should be no grudge in providing the
same. The doors should be opened for obligatory licensing involving the domestic enterprises in the production of patented drugs. The
suggestions made in this Paper are within the framework of the TRIPS Agreement. Judicious and careful implementation of TRIPS is needed
for its smooth application and balancing of rights and obligations of the patent holder in a manner conducive to social and economic
welfare as stipulated in Article 7 of TRIPS Agreement. India can play an effective role in the region about the availability of generic
drugs by the pharmaceutical industry only after the ignored issues are provided through further amendments to the Patents Act 1970.
B K Keayla
B K Keayla is retired as Commissioner of Payments. Presently he is Convenor, National Working Group on Patent Law and
Trustee and Secretary General of Centre for Study of Global Trade System & Development.
•
Vol #4, Issue #2, Combat Law
•
Trade
•
Printer friendly version
![]() Amended Patents Act: A critique
Amended Patents Act: A critique
India's recent position on patents means that it is going to make its products extremely expensive and out of reach for
its own people and their brethren within the developing world.
B K Keayla
critiques the direction the Indian government is taking with the new patent laws.

Combat Law, Vol. 4, Issue 2 -
TRIPS is one of the most contentious agreements of the WTO which has been debated world-wide in the developed and developing countries
and also in important international institutions. In the recent past, a number of studies and research papers by important institutions
and eminent economists have been published about the implications of TRIPS on the developing countries. These studies can serve as useful
guide for safeguarding the interest of the industry and public. However, all the member countries of the WTO including India are under
binding commitment to implement the TRIPS provisions in their national patent laws.
![]() The Patents (Second Amendment) Bill 1999 was referred to the Joint Select Committee of Parliament and it submitted its report in December
2001 with a few notes of dissent. The Doha Declaration on TRIPS and Public Health of November 2001 which clarified flexibilities and
freedom available to member countries in formulating their law was, however, not considered by the Committee and certain safeguards
possible were not provided by the committee in their report. The revised Bill as amended by the Joint Parliamentary Committee was debated
in the Rajya Sabha and 18 amendments were moved by important Members of Parliament. The debates in both the Houses indicate that there
was some understanding with the then concerned minister about the issues raised by the members of Parliament for taking them into
consideration in the final amending Bill introducing the product patent regime for all industrial sectors. The amendments proposed by the
MPs were either negated or withdrawn.
The Patents (Second Amendment) Bill 1999 was referred to the Joint Select Committee of Parliament and it submitted its report in December
2001 with a few notes of dissent. The Doha Declaration on TRIPS and Public Health of November 2001 which clarified flexibilities and
freedom available to member countries in formulating their law was, however, not considered by the Committee and certain safeguards
possible were not provided by the committee in their report. The revised Bill as amended by the Joint Parliamentary Committee was debated
in the Rajya Sabha and 18 amendments were moved by important Members of Parliament. The debates in both the Houses indicate that there
was some understanding with the then concerned minister about the issues raised by the members of Parliament for taking them into
consideration in the final amending Bill introducing the product patent regime for all industrial sectors. The amendments proposed by the
MPs were either negated or withdrawn.
![]() As regards the Doha Declaration the members right to protect public health has been recognized. It has also been clarified that the
members have right to grant compulsory licences and freedom to determine the grounds therefor. Further it has also been clarified that
each provision of TRIPS could be read in the light of its objectives (in Article 7) and principles (in Article 8). If all the above
flexibilities had also been applied in the amending process , the public interest about the availability and affordability would have
been protected to a considerable extent.
As regards the Doha Declaration the members right to protect public health has been recognized. It has also been clarified that the
members have right to grant compulsory licences and freedom to determine the grounds therefor. Further it has also been clarified that
each provision of TRIPS could be read in the light of its objectives (in Article 7) and principles (in Article 8). If all the above
flexibilities had also been applied in the amending process , the public interest about the availability and affordability would have
been protected to a considerable extent.
![]() The stipulation in Article 31(b) of TRIPS is a very important provision for substantive role by the domestic enterprises. In fact this
provision is heart and soul of TRIPS for developing countries. This stipulation in TRIPS is almost similar to 'licences of right'
provision in original Patents Act 1970. Even meeting of export demands would be possible only when such a provision is there in the law
as there should be some enterprise already producing for domestic demand. Only the producing enterprises can meet the export demand.
It would be pertinent to mention that it takes almost three to four years to develop technology and stabalise the product. As such an
enterprise already in production of the relevant patented products has to be there in the country to respond to export demand. In view of
these consideration and the examples of other countries it is extremely important that provision is made through a new Section 84A as
follows :
The stipulation in Article 31(b) of TRIPS is a very important provision for substantive role by the domestic enterprises. In fact this
provision is heart and soul of TRIPS for developing countries. This stipulation in TRIPS is almost similar to 'licences of right'
provision in original Patents Act 1970. Even meeting of export demands would be possible only when such a provision is there in the law
as there should be some enterprise already producing for domestic demand. Only the producing enterprises can meet the export demand.
It would be pertinent to mention that it takes almost three to four years to develop technology and stabalise the product. As such an
enterprise already in production of the relevant patented products has to be there in the country to respond to export demand. In view of
these consideration and the examples of other countries it is extremely important that provision is made through a new Section 84A as
follows :
Combat Law, Volume 4, Issue 2
June-July 2005
(published July 2005 in India Together)
•
Volume 4, Issue #2, Combat Law
•
Laws
•
Trade
•
Feedback :
Tell us what you think of this page.
